Abstract

Introduction: Individuals with cancer are at high risk for COVID-19 but risk of complications including severe disease, TE and mortality have not been fully evaluated prospectively. We prospectively assessed risk of post-Covid TE, severe disease and mortality and whether hemostatic or inflammatory biomarker assays could predict poor outcomes.
Methods: NCCAPS is a longitudinal cohort study of patients on treatment for hematologic or solid malignancy with acute COVID-19, enrolled via three NCI trials networks involving 261 sites across 43 states, District of Columbia, Puerto Rico and Canada between May 21, 2020 and February 1, 2022.Adult patients within 14 days of initial positive SARS CoV-2 test and receiving anticancer treatment within 6 weeks or a history of stem cell transplant or CAR-T cell therapy were eligible for these analyses. Outcomes included severe COVID-19 (defined as hospitalization for COVID-19 or death within 30 days of first positive test), TE (defined as any venous or arterial TE within 30 days of first positive test), and 90-day all-cause mortality. A biomarker sub-cohort was restricted to patients with ≥ 1 biomarker values available by centralized biospecimen testing (Quest Diagnostics) for levels of C3, C4, D-dimer, fibrin degradation products (FDP), factor VIII (FVIII), high sensitivity-C-reactive protein (hs-CRP), and von Willebrand factor (vWF), from plasma or serum collected within 14 days of the patient's first positive test. Statistical analyses include Fisher's exact tests and corresponding estimated ORs, logistic regression (univariate for each biomarker, and multivariate for each biomarker adjusting for sex, race/ethnicity, age, malignancy category, and use of therapeutic anticoagulation ±14 days of positive test). Survival analyses included Kaplan-Meier curves stratified by presence vs absence of elevated baseline biomarker and both uni- and multivariable Cox proportional hazards analyses adjusting for the same patient characteristics. All analyses were conducted using R version 4.1.1, with tidyverse package version 1.3.1 and survival package version 3.2.11.
Results: The study population comprised 1,619 patients; 344 (21%) were hospitalized within 30 days of first positive COVID test. The biomarker subcohort included patients (60 hospitalized). Approximately one-third of patients had hematologic malignancies; one- third metastatic solid tumors and one-fifth non-metastatic solid tumors. Of the study cohort, 373 patients (23%) had severe COVID-19; 48 (3.0%) had TE within 30 days (2.7% VTE, 0.2% ATE)); 90-day mortality was 8% (N=129).
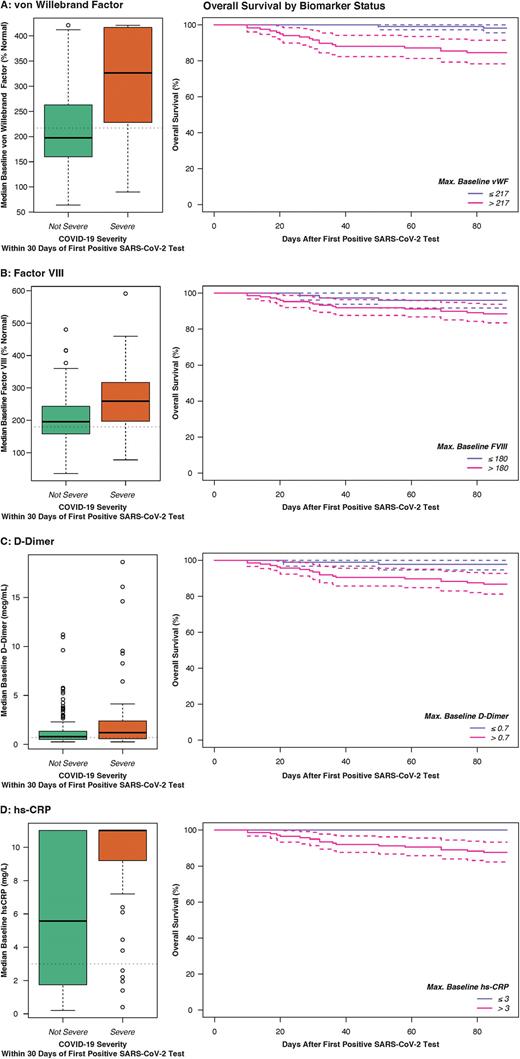
Baseline vWF [OR 1.18 per 10% above normal as continuous variable, p=0.01] and FVIII [OR 1.16 per 10% above normal, p=0.007] were associated with increased risk of TE. Elevated baseline vWF [adjusted OR (aOR) 8.02 as categorical variable, p=0.0001; aOR 1.10 per 10% increase as continuous variable, p=0.0001 respectively] and FVIII [aOR 3.56, p=0.0009 as categorical; 1.08 per 10% increase, p=0.001] were also strongly associated with risk of severe COVID-19, as were D-dimer [aOR 2.455 as categorical, p=0.03; 1.15 per mcg/dL increase, p=0.09] and hsCRP [aOR 3.41 as categorical, p=0.05; 1.23 per mg/L, p=0.001]. All four biomarkers were also associated with increased 90-day mortality [vWF adjusted HR (aHR) 12.4 as categorical, p=0.02, 1.13 per 10% increase, p=0.0001; FVIII aHR 3.63 as categorical, p=0.09, 1.06 per 10% increase, p=0.008; D-dimer aHR 11.1 as categorical, p=0.02, 1.36 per mcg/dL increase, p=0.00001; hs-CRP aHR 1.41 per mg/L increase, p=0.02] (Figure). 90-day survival in patients with elevated vWF and FVIII was 84.6% (95% CI 78.2-91.4) and 88.4% (95% CI 83.5-93.8) respectively, compared to 98.1% (95% CI 95.6-100) and 96.1% (95% CI 91.8-100) in patients without elevated levels.
Conclusions: In a multicenter prospective longitudinal cohort study, patients with cancer and COVID-19 had a 3% 30-day risk of TE. Hemostatic biomarkers measured at baseline, particularly vWF and FVIII were predictive of TE, severe COVID-19 and death. These novel findings provide insight into pathophysiologic mechanisms and may be used in clinical settings to identify patients with COVID-19 undergoing treatment for cancer who are at high risk for poor outcomes.
Disclosures
Khorana:Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; sanofi: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Anthos: Consultancy, Honoraria. Pergam:Global Life Technologies: Research Funding; Merck: Other: Clinical trial participant. Rini:BMS: Consultancy, Honoraria; Debiopharm: Consultancy, Honoraria; Pfizer: Research Funding; Hoffman La Roche: Research Funding; Incyte: Research Funding; Astra Zeneca: Research Funding; SeaGen: Research Funding; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Aveo: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Eisai: Consultancy, Honoraria; EUSA: Consultancy, Honoraria; Allogene Therapeutics: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; BMS: Research Funding; Mirati: Research Funding; Merck: Research Funding; Surface: Research Funding; Arrowhead: Research Funding; Immunomedics: Research Funding; Alkermes: Consultancy, Honoraria; Arrowhead: Consultancy, Honoraria; Corvus: Consultancy, Honoraria; Aravive: Consultancy, Honoraria; Aravive: Research Funding; Exelixis: Research Funding; Janssen: Research Funding; Pionyr: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal